Featured
- Get link
- X
- Other Apps
Which Compound Produces An Acidic Solution When Dissolved In Water
Which Compound Produces An Acidic Solution When Dissolved In Water. Well, there are lots of compounds that react with water to produce acidic solutions. Thus, ( n h 4 c l) will give an acidic solution on dissolving in water.
Well, there are lots of compounds that react with water to produce acidic solutions. Slippery feel, turn litmus blue, and react with acids. Thus, 0.1 mol nah 2 po 4 and 0.1 mol na 2 hpo 4 in 1.0 l of pure water will produce a buffer solution.
Basic Compouds Are Compounds That When Dissolved In Water, Gives A Solution With Hydrogen Ion Activity Greater Then In Pure Water A Ph More Than 7.0, Such As Ammonia And Sodium Hydroxide.
A) 0.0576 n b) 0.293 n N h 4 c l produces acidic solution 2. According to this, na 2 hpo 4 is the sodium salt of nah 2 po 4 which is a weak acid.
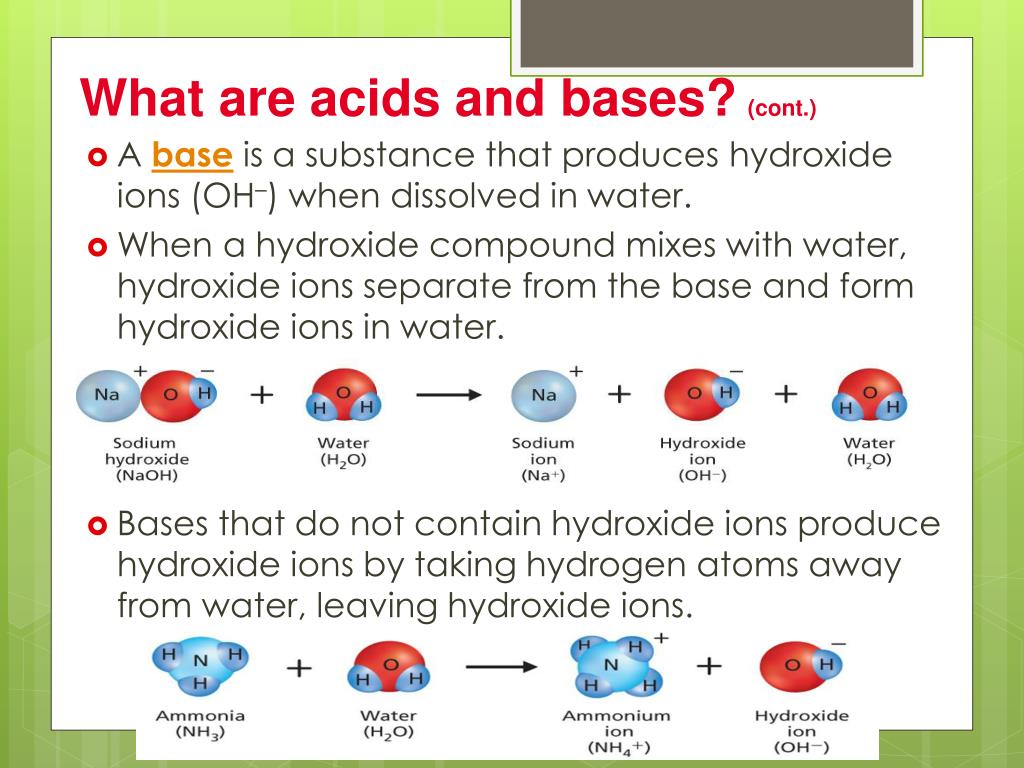
A Base Is A Compound That Produces Hydroxide Ions When Dissolved In Water.
(a) what is the ph of 0.65 m potassium formate, hcook? Acids are known to turn blue litmus red. Well, there are lots of compounds that react with water to produce acidic solutions.
An Acid Is Basically A Molecule That Can Donate An H + Ion And Can Remain Energetically Favourable After A Loss Of H +.
This is because the water molecule readily takes up the liberated h + ion from the acids and forms a hydronium ion. A) kclo4 b) ca(no3)2 c) nh4cl d) kcl e) naf. This makes option (c) correct.
N A C N Produces Basic Solution 4.
K h s o 4 produces acidic solution because the neutral salt of strong base k o h and strong acid h 2 s o 4 is k 2 s o 4 3. 71) what is the normality of a solution prepared by dissolving 75.0 g citric acid, a triprotic acid with molar mass of 192.14 g, in water to make 250. Therefore, the correct answer is option (b) note:
A) So2 B) Na2O C) Co2D) Cao E) No Correct Answerc) Is Not The Only Correct Answer Here.
The salt, ca (cn)2, would give an aqueous solution that is (a) acidic (b) basic (c) neutral (d) none…. Which one of the following compounds would produce an acidic solution when dissolved in water? “an acid is a substance that contains hydrogen and ionizes to produce hydrogen ions in aqueous solutions.
Comments
Post a Comment